Innovating in the world of oncology through RNA therapeutics
December 2, 2024
.png)
An introduction to Flamingo Therapeutics, its foundation and VIB Roots
Flamingo Therapeutics, an innovative VIB spin-off company, is spearheading the development of antisense technology in oncology with its lead asset in Phase 2 development being the furthest advanced antisense molecule in oncology. VIB, as co-founding shareholder, has supported the company from inception on through direct R&D collaborations, with strategic advice and support on the company's board of directors.
Historically, the company focused on using antisense technology to target long non-coding RNA (lncRNA) which has previously been dismissed as "junk" RNA due to its non-protein-coding nature. However, lncRNAs are now recognized for their critical role in cellular biology. Today, the company has a pipeline of antisense-based molecules that target both lncRNA as well as classical protein coding RNAs.
Flamingo Therapeutics stands out in the oncology landscape due to its unique focus on targeting cancer cells as well as the tumor microenvironment through antisense based therapies. Danvartirsen, its lead asset, is designed to inhibit STAT3, a transcription factor implicated in both solid tumors (e.g., head and neck cancer) and blood cancers such as acute myeloid leukemia (AML). By targeting the tumor stroma and immune suppressor cells, danvartirsen works synergistically with immune checkpoint inhibitors to enhance the immune system’s ability to fight cancer. This dual approach – stimulating the immune response while also modifying the tumor environment – holds great promise for patients who may have developed resistance to immunotherapies.
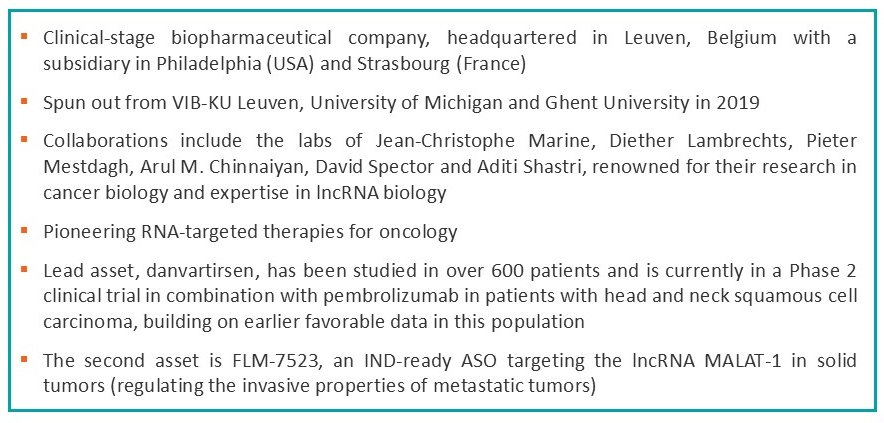
Strategic Collaborations and Clinical Development
In its early days, Flamingo focused on validating lncRNAs as therapeutic targets, supported by its deep academic roots. A pivotal moment came in 2021 when the company entered a strategic collaboration with Ionis Pharmaceuticals, a California-based biotech giant. Ionis, a major player in the RNA therapeutics space, had two oncology assets in its pipeline which were subsequently licensed to Flamingo and are currently being developed as danvartirsen and FLM-7523. This partnership significantly elevated Flamingo’s standing in the biopharmaceutical industry, with Ionis becoming a shareholder.
The next transformative event occurred in 2023 when Flamingo merged with Dynacure, a company based in Strasbourg that shared a similar focus on antisense oligonucleotides (ASOs). The merger bolstered Flamingo’s clinical development pipeline and brought in critical funding, with the backing of regional as well as international investors. The combined resources allowed Flamingo to initiate a Phase 2 clinical trial for its lead asset, danvartirsen, and transitioned Flamingo from a research-focused organization to a clinical-stage company.
_Linkedin.jpg)
“Danvartirsen is now in a Phase 2 clinical trial for head and neck cancer. That’s a major milestone for our team,” says Stephane van Rooijen, CEO of Flamingo Therapeutics. “It demonstrates our company’s ability to advance promising assets through the clinical development process and underlines our commitment to making a meaningful impact for oncology patients.”
The Role of the CMO in Shaping Clinical Strategy
Drew Denker, Flamingo’s Chief Medical Officer (CMO; based in Philadelphia, USA), joined the company following its merger with Dynacure in 2023. His dual role as CMO and head of the research team underscores his critical position in guiding Flamingo through the complex domain of oncology drug development. In addition to overseeing the clinical trials, Drew and his team actively engage with the physicians running the trials and work closely with the company’s external collaborators, ensuring that Flamingo maintains direct involvement in every aspect of its clinical operations.

“For us, it’s essential to not just rely on contract research organizations to run trials but also to build direct relationships with investigators. This approach fosters better communication, faster decision-making, and ensures that Flamingo remains nimble in an industry where the speed and efficiency of clinical development can determine a company’s success,” emphasizes Drew Denker. “Our strategy is clear: keep the team lean but strategic, make data-driven decisions, and ensure that every step taken in the clinical trial process serves the ultimate goal of bringing innovative treatments to patients.”
The decision to run a stand-alone Phase 2 trial for danvartirsen, rather than rushing to a Phase 2/3 combined trial, is one example of Flamingo’s thoughtful approach to clinical development. While some companies might rush to large trials based on limited early data to expedite timelines, Flamingo has opted for a more measured approach.
“The company’s focus is not only on regulatory approval but also on generating robust data that will convince both physicians and payers of the therapeutic’s value,” adds Drew. “This strategy is particularly important in Europe, where healthcare reimbursement decisions often hinge on the quality of life and overall benefit that a treatment provides, beyond just clinical efficacy.”
In addition to his clinical responsibilities, Drew is also shaping the future of Flamingo's research team. Based in Leuven, this agile group of 8-10 researchers is responsible for conducting in vivo and in vitro experiments, as well as translational research that supports Flamingo’s clinical programs. Drew has fostered a culture of collaboration, ensuring that the research team understands the impact of their work on real-life clinical outcomes—a transparency that is often lacking in larger organizations.
“Flamingo’s culture of decision-making and transparency ensures that every team member, from research to clinical operations, is aligned with the company’s mission of transforming cancer treatment,” concludes Drew.
A Promising Future with Strategic Growth
Beyond its lead asset, Flamingo is also exploring additional indications for danvartirsen and developing FLM-7523, an IND-ready ASO targeting the lncRNA MALAT-1 in solid tumors. While the company's primary focus remains on oncology, its leadership has expressed openness to exploring partnerships in other therapeutic areas if compelling opportunities arise. An example of a partnership between Flamingo and academic institutions in Belgium is the collaboration among Flamingo and researchers at KU Leuven and Ghent University to identify new oncology targets for ASO therapeutics. The collaborations are funded by a VLAIO1 grant obtained by Flamingo.
Stephane van Rooijen: “Flamingo Therapeutics is keen to build on its strong relationships with both academic institutions and industry partners, and is actively seeking additional fundraising opportunities in the US and Europe, with the ultimate goal of bringing its unique RNA-based therapies to patients in need.”
From its humble beginnings as a spin-off of VIB to its current status as a clinical-stage company with promising assets in development, Flamingo is poised for success. For investors and business partners, this is a company worth watching closely as it has a strong foundation in science, a robust clinical pipeline, and a clear vision for the future.
1 VLAIO: Flanders Innovation & Entrepreneurship
.jpg)